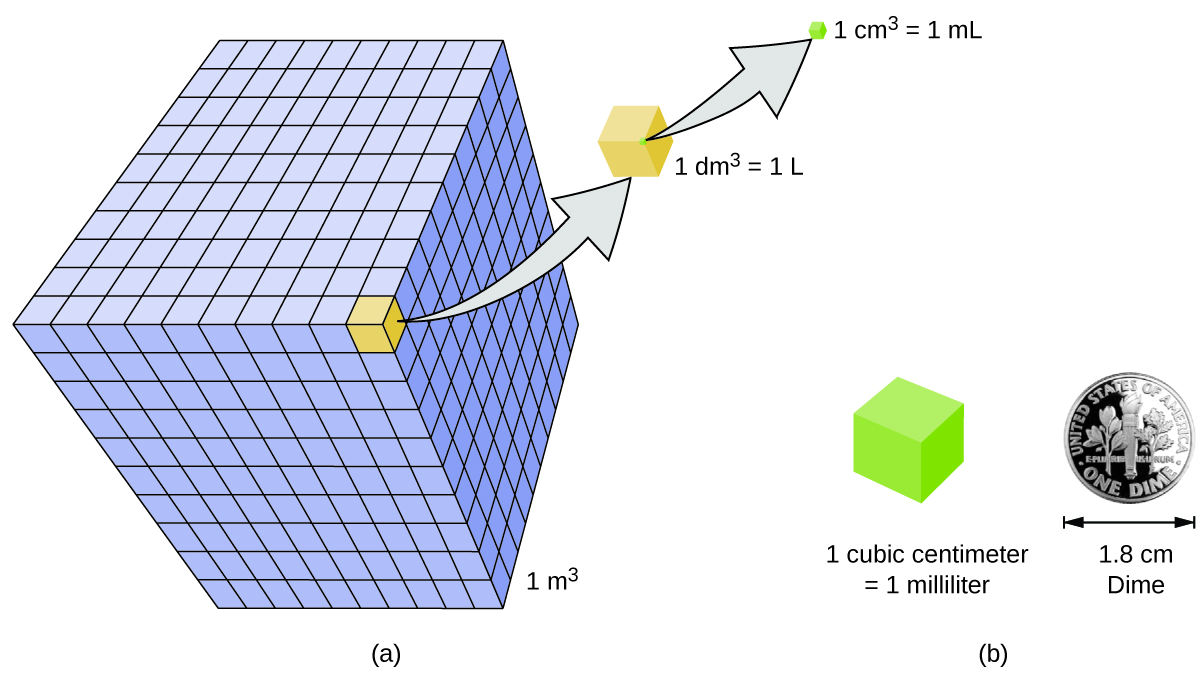
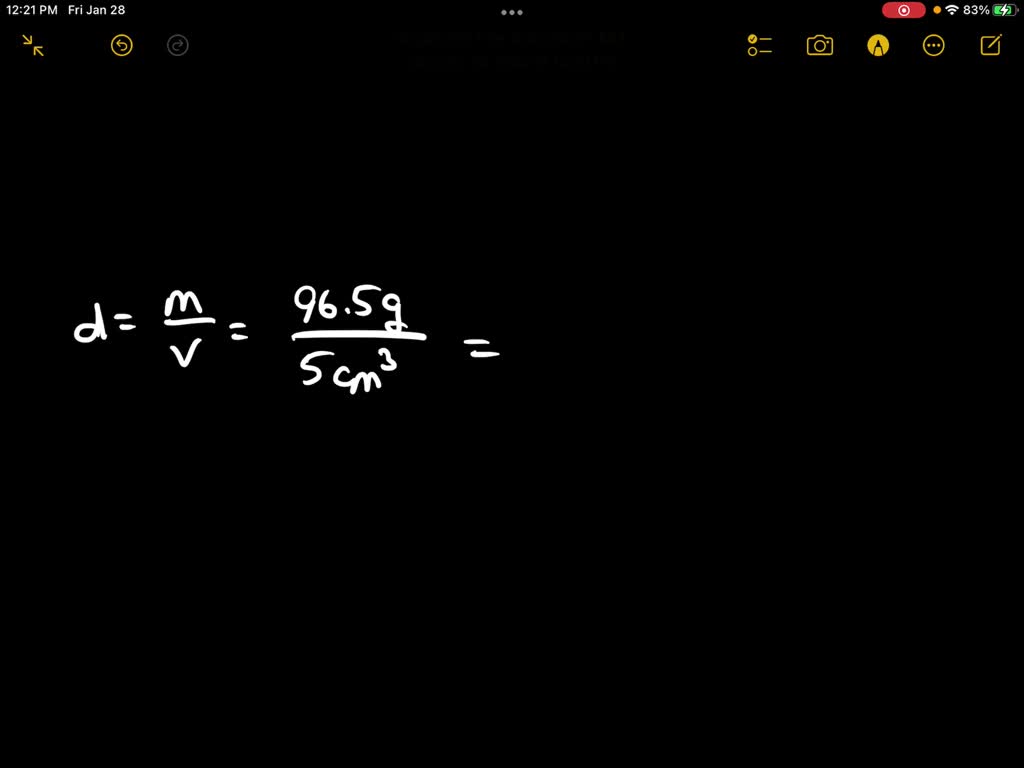
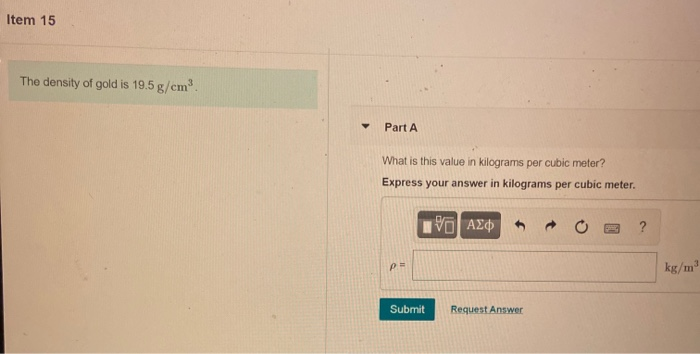
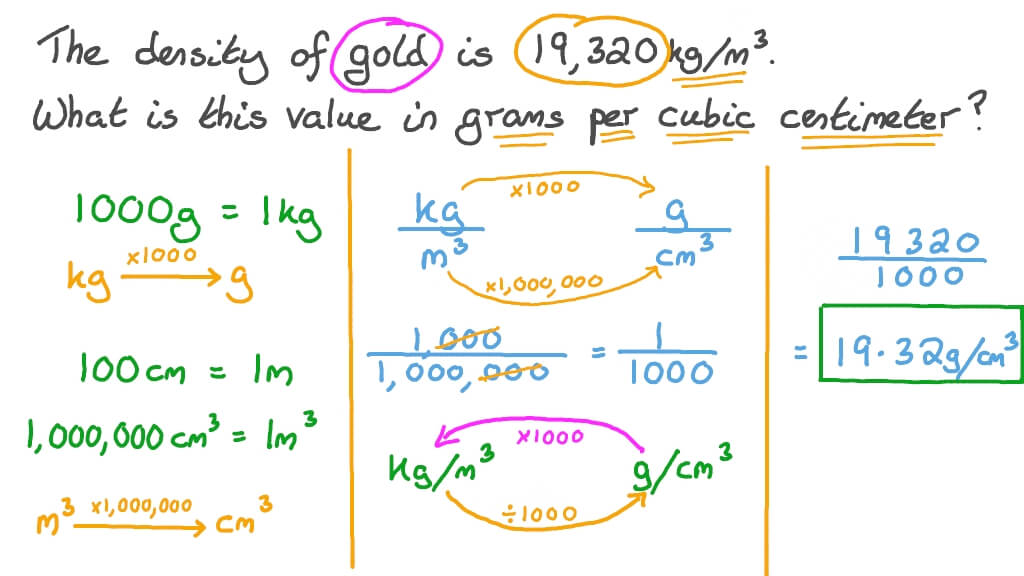
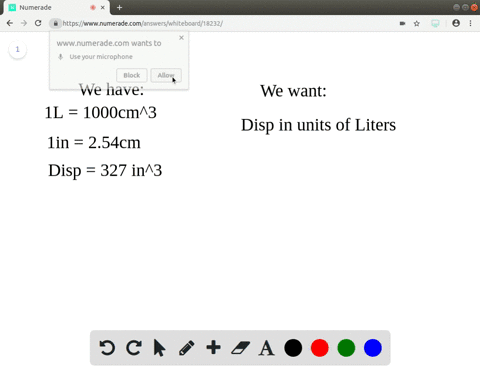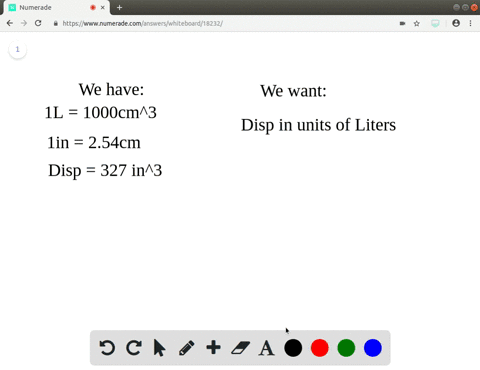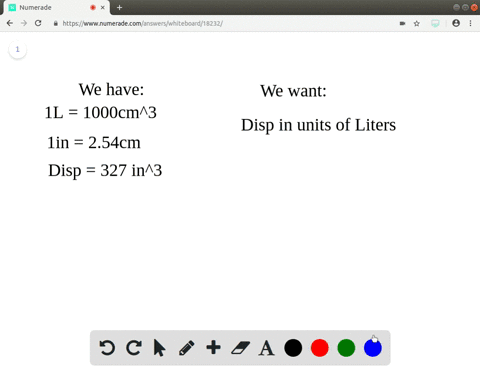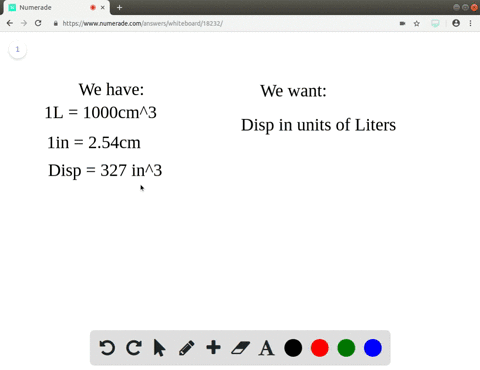
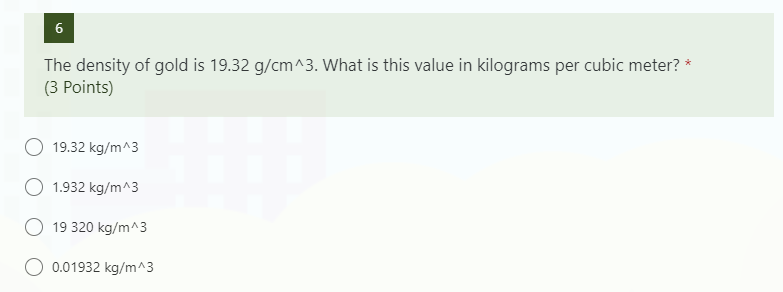
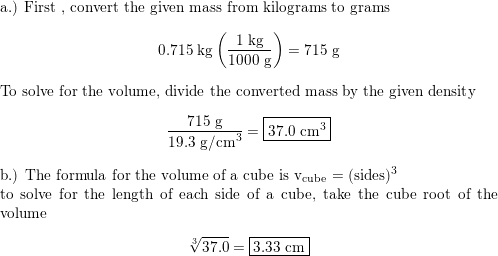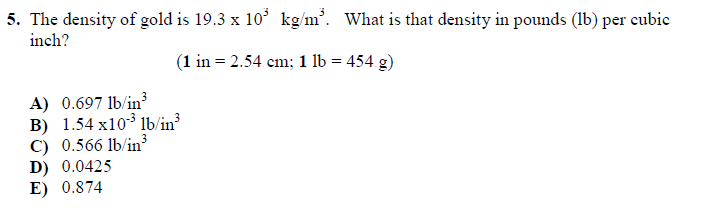A piece of pure gold of density `19.3 g cm^(-3)` is suspected to be hollow inside. It weighs 38.250 - YouTube
The density of gold is 19.300 kg/M3. What is the mass of a gold cube with a side length of 20.15 cm? - Quora

Given mass number of gold `= 197`, Density of gold `= 19.7 g cm^(-1)`. The radious of the gold a... - YouTube

HW3_solutions - 4.4 Calculate the number of vacancies per cubic meter in gold (Au) at 900C. The energy for vacancy 3 formation is 0.98 eV/atom. | Course Hero
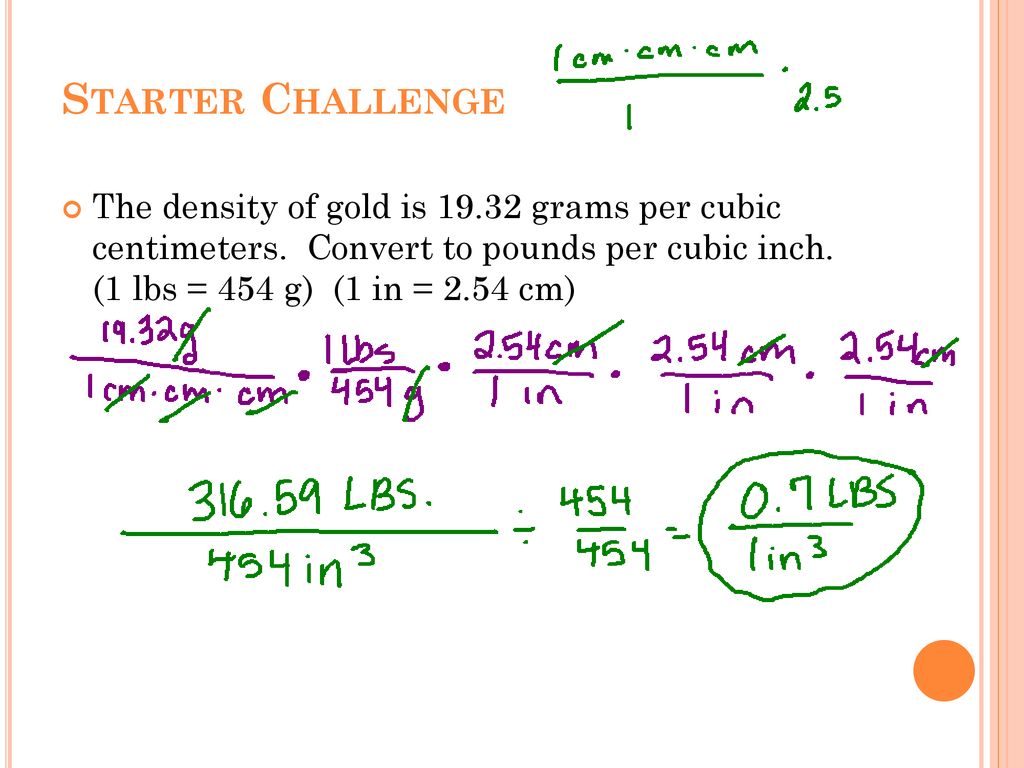
Starter Challenge The density of gold is grams per cubic centimeters. Convert to pounds per cubic inch. (1 lbs = 454 g) (1 in = 2.54 cm) - ppt download


:max_bytes(150000):strip_icc()/GettyImages-139820160-58b599a75f9b5860467cbd20.jpg)